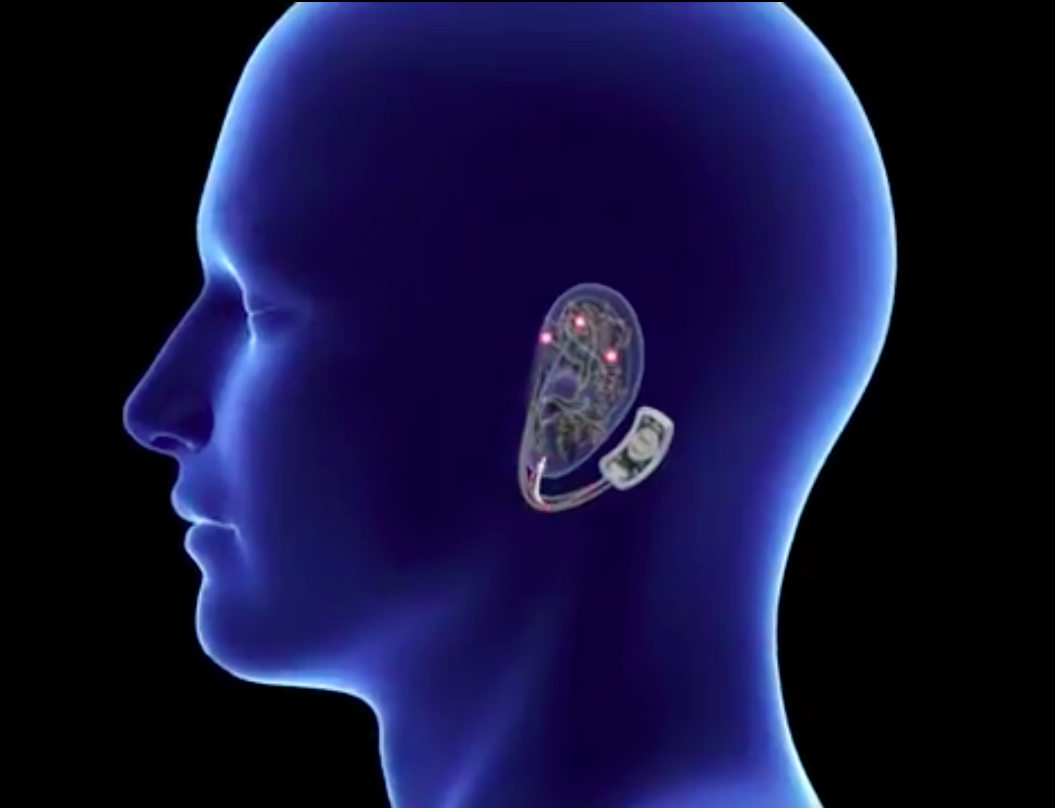
Detoxing from opioids can be an intense physical process so daunting that some people continue to use drugs to avoid it.
The Bridge device—which is placed behind a person’s ear and delivers electrical signaling—promises to block withdrawal symptoms, making the early stages of detox more manageable.
However, an investigation by NPR and Side Effects Public Media raises questions about how the device was introduced to the market and about the standards of a study that helped lead to the recent FDA-approval of the product.
“For the sake of patients, I do hope the Bridge works,” Andy Chambers, an addiction psychiatrist in Indianapolis, wrote in a letter published in the Indianapolis Star in November. “But for me to use it in regular practice, don’t show me a sales job, or the enthusiasm of lobbyists, politicians or prosecutors; just give me the solid science.”
The Bridge, which was designed by Innovative Health Solutions, was approved for treating pain relief in 2014. Soon after, Arturo Taca, an addiction psychiatrist who practices near St. Louis, helped the company adapt the Bridge to treat withdrawal symptoms. For at least a year, Innovative Health Solutions has been marketing the device to treat opioid withdrawal.
Although off-label use is fairly common in the healthcare field, promoting a product as such is not actually allowed, said one expert.
“They are not allowed to promote off-label use period, whether it’s to a physician, whether it’s to a judge, whether it’s to just a random person in the public,” said Basia Andraka-Christou, a health policy researcher at the University of Central Florida.
Last November, Innovative Health Solutions received FDA approval for the device to treat opioid withdrawal based on a study into the device’s efficacy. Initially, the researchers said it was a “retrospective study” which simply reviewed existing medical data. But according to NPR’s Jake Harper, the research was more problematic than it appeared.
“It seems that they instead conducted a clinical trial that skirted FDA rules and ethical norms, using vulnerable people suffering from addiction as test subjects,” Harper claimed.
Arturo Taca—who has applied for a patent on his treatment method using the Bridge and thus could profit from its approval—provided much of the data for the study from his clinic, Harper noted.
“This is an enormous, astonishing, unbelievable conflict of interest,” said Jake Sherkow, who teaches patent and FDA law at New York Law School. “It casts significant doubt on the results of the study.”
In addition, the structure of the study, which allowed patients to get the device and then check in five days later, was not up to scientific standards, others claim.
“Most studies of withdrawal are done in residential settings, where people are under pretty continuous monitoring and observation,” said George Bigelow, a professor who heads an institutional review board (IRB) at Johns Hopkins University.
The study also did not report how many people dropped out of the research.
“If you treat 10 people successfully and crow about it, but 90 people dropped out… that’s pivotal,” said David Clark, who oversees an IRB that supervises research at the Medical College of Wisconsin.
Still, Innovative Health Solutions stands by the product.
“I’m still looking for the first device that didn’t work. I keep asking and no one can find it,” said Brian Carrico, now the president of Innovative Health Solutions. “If it was placebo, it’s 100% placebo. That’s the best placebo I’ve ever seen.”
