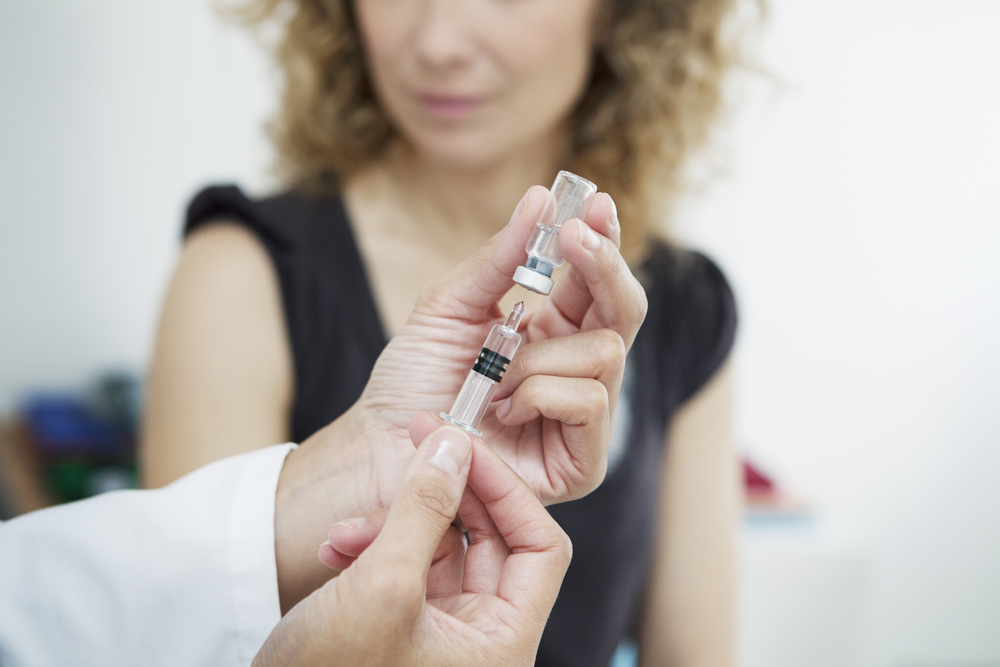
Indivior, the company behind Suboxone, has won approval for an injectable version that could reduce the potential for abuse.
According to STAT, the FDA has approved Sublocade, an injectable version of their widely-used opioid addiction treatment drug, Suboxone, which could potentially reduce relapses as well as the stigma that surrounds Suboxone tablets. Because it is administered by a health professional monthly, the potential for abuse is greatly reduced, making the treatment seem more legitimate.
“It’s potentially a game changer,” said Dr. Andrew Kolodny, co-director of opioid policy research at Brandeis University. “This could become first-line [medication] for opioid addiction. It could open up opportunities for getting more patients on buprenorphine.”
The FDA advisory panel voted to approve the drug 18 to 1, a move that some hope can help curb the opioid crisis. Sublocade could be “part of our ongoing work in supporting the treatment of those suffering from addiction to opioids,” said Scott Gottleib, FDA Commissioner, in a press release. “We’ll continue to pursue efforts to promote more widespread use of existing, safe and effective FDA-approved therapies to treat addiction.”
The approval comes after an open-label clinical trial and a randomized controlled clinical trial with 848 adults who suffered from opioid use disorder who had already started a Suboxone regimen. Urinalysis tests found that those on Sublocade went “more weeks without positive urine tests or self-reports of opioid use” than those who were treated with a placebo. A higher proportion of patients using Sublocade also showed “no evidence of illicit opioid use throughout the treatment period.”
Sublocade is administered via an abdominal injection by a health professional in conjunction with daily doses of sublingual buprenorphine. Unlike Vivitrol, an injectable extended-release naltrexone, Sublocade will not require patients to detox first.
“Buprenorphine gives a stimulation of the opioid receptors, and that means better control of cravings without experiencing subclinical withdrawal,” said Dr. Kolodny.
Due to the opioid crisis, there has been more Medicaid spending on opioid addiction treatment prescriptions than ever—more than doubling from 2011 to 2016. Without the requirement of detox, Sublocade could see more widespread use, including inside the criminal justice system. Judges and wardens have so far been hesitant to bring in buprenorphine tablets because of their potential for trade and abuse.
“In the criminal justice setting, they’ve been very reluctant to provide [daily doses of] buprenorphine in prisons,” said Dr. Nora Volkow, director of the National Institute of Drug Abuse. “The question now is how will they respond to [the fact that] extended-release buprenorphine cannot be diverted.”
